top of page
The ELEMENTS REIMAGINED

Name: Strontium
Atomic Symbol and Mass Number: Sr [38]
Electron Configuration of the Element: [Kr] 5s
Atomic Radii: 255 pm
Electron Affinity: 0
Oxidation States: +2, +1
Electronegativity value: 0.95
Elemental Group: Alkaline Earth Metal
Uses and properties:
-
A soft, silvery metal
-
Turns yellow when cut
-
Finely powdered strontium metal is sufficiently reactive to ignite spontaneously in air
-
Reacts with water rapidly but not violently
-
Burns in a crimson flame
-
Production of cathode ray tubes for color televisions
-
Refining zinc
-
Production of ferrite ceramic magnets
-
Crimson color in fireworks and flares
-
Stronium chloride in toothpaste for sensitivity
-
Development of the most accurate atomic clock
Lewis diagram:
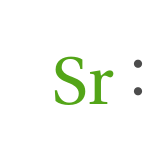
2
bottom of page